Insomnia is a significant condition that affects more than just sleep. People who suffer from the problem are at increased risk for mental problems, are less productive, and are more accident-prone. For example, experts link both the Challenger and Chernobyl disasters to lack of sleep. Insomnia is also a factor in the dysregulation of several body systems and can lead to several common chronic conditions such as type 2 diabetes, heart disease, coronary artery disease, obesity, and depression.
For example, experts link heart rate variability (HRV), part of autonomic regulation, to insomnia and overall higher mortality.
Research continues to develop new ways to reduce symptoms and help patients get a good night’s rest. In this pilot clinical trial, published in Global Advances in Integrative Medicine and Health, authors tested a noninvasive closed-loop system that uses precision-guided technology to impact neural circuits at points that promote sleep.
These systems are a type of acoustic stimulation, where the machine rapidly echoes brain waves in real-time as audible tones. This acoustic stimulation at certain brain waves has been shown to effect the autonomic nervous system and promote restful sleep.
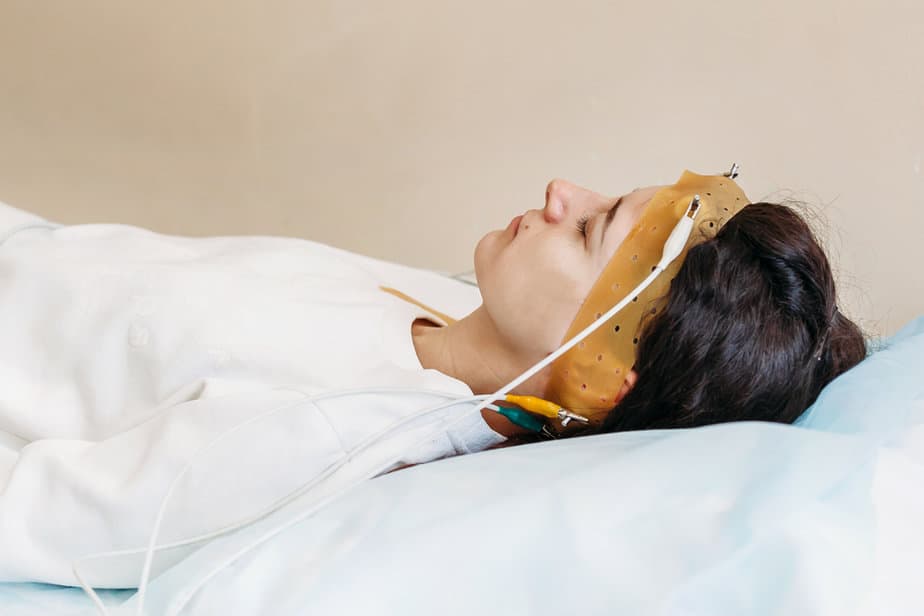
In a previous randomized, placebo-controlled research study, High-resolution, relational, resonance-based electroencephalic mirroring (HIRREM®, registered trademark of Brain State Technologies LLC, Scottsdale, AZ) showed that this technology was safe, effective, and well-tolerated. However, it has some limitations including being operator-dependent and taking a relatively long time.
The authors of this new research upgraded the system at Wake Forest University to make it almost completely automated and rebranded it as Cereset ResearchTM (CR). Unlike the original system, CR generates tones by software-guided, algorithmic analysis of real-time brain electrical activity.
The Study
This study evaluated the preliminary efficacy of their standardized, more scalable, and less-time intensive version of this brainwave echoing technology. They enrolled 22 men and women over the age of 18. Out of the enrolled participants, they gave 10 the intervention and 12 a mock control. The study used ten sessions of either interventions or mock controls (randomly generated tones) for four weeks.
They supplied the tones with easy to control ear buds.
Both groups had 1 participant drop out during the trial. They collected their initial results within two weeks of the participant’s final session and repeated these measurements 4-6 weeks later.
This ended the initial study. Then, the authors allowed the patients in the mock control group to take the therapy. Seven patients participated and repeated the trial with the intervention.
Additionally, individuals received continuous recordings of blood pressure and heart rate to analyze measures of autonomic cardiovascular regulation.
Results
The main measurements were taken as surveys that measured various aspects of sleep and sleep-related conditions. The primary result was the Insomnia Severity Index (ISI) score where higher scores indicate more insomnia. The control group showed a 4.69-point reduction in ISI score while the intervention group’s ISI score dropped by 7.27 points.
Although promising, this result is not statistically significant, most likely due to the small sample size of the trial. In comparison, the intervention group’s sleep quality, measured by the Pittsburgh Sleep Quality Index (PSQI), was significantly improved, dropping by 5.74 points.
The authors also measured indicators for anxiety, depression, and perceived stress. All of these values seemed to improve with the intervention but once again were not statistically significant.
The authors also saw significant improvements in HRV measurements at the end of the study compared to measurements taken at the beginning.
Overall the results show that this neurotechnology improves autonomic function and insomnia.

Summary
The study has some limitations, most notably the small sample size as appropriate for a pilot study. The authors also note that participants had a range of insomnia severity, which may have introduced variability. Finally, there was a lack of diversity in participants (most were older white females), reducing the study’s generalizability.
By demonstrating the feasibility of automatic, scalable technology experts hope that these types of alternative techniques can be made more widely available, especially for people with conditions such as post traumatic stress disorder. They may be especially important for patients who do not wish to rely on medication or for those whose other therapies have not been successful.
Citation: Tegeler CL, Munger Clary H, Shaltout HA, Simpson SL, Gerdes L, Tegeler CH. Cereset Research Standard Operating Procedures for Insomnia: A Randomized, Controlled Clinical Trial. Global Advances in Integrative Medicine and Health. 2023;12. doi:10.1177/27536130221147475
