Table of contents
Nebula Genomics DNA Report for Parkinson’s Disease
Is Parkinson’s genetic? We created a DNA report based on a study that attempted to answer this question. Below you can see a SAMPLE DNA report. To get your personalized DNA report, purchase our Whole Genome Sequencing!

| This information has been updated to reflect recent scientific research as of May 2021. |
What is Parkinson’s?
Parkinson’s disease or PD is a result of the slow, progressive loss of nerve cells. The characteristic symptoms include akinesia (reduction or loss of voluntary movement), rigor, and tremors. Currently, there is no cure for this degenerative condition, although the symptoms are treatable.
Parkinson’s disease is characterized by the predominant loss of dopamine-producing neurons in the substantia nigra region in the brain. The lack of the neurotransmitter dopamine ultimately leads to a reduction in the activating effect of the basal ganglia on the cerebral cortex and thus to the noticeable movement disorders.
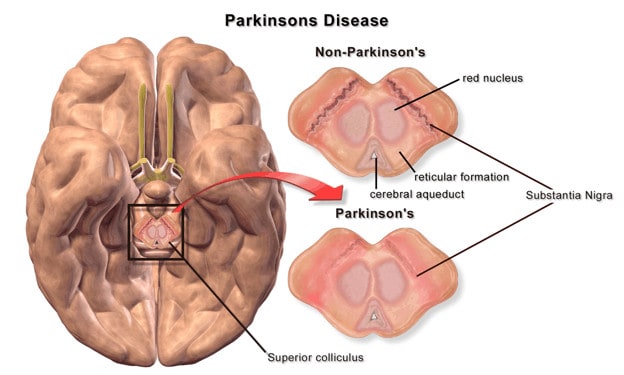
There are a number of classifications of Parkinson’s disease based on disease origin and progression. These categories include:
- Idiopathic Parkinson’s syndrome (IPS) (most frequent)
- Familial Parkinson syndrome (genetic factors, hereditary forms, rare, named after the respective gene locus (i.e., PARK1)
- Symptomatic (secondary) Parkinson’s syndromes
- Drug-induced (i.e., neuroleptics with dopamine antagonism)
- Vascular parkinsonism, as in cerebral microangiopathy (Binswanger’s disease)
- Post-traumatic (i.e., boxer’s encephalopathy)
- Toxin-induced (i.e., carbon monoxide, manganese, MPTP)
- Inflammatory (e.g., after encephalitis lethargica, also in diffuse pathogen-related brain conditions such as advanced HIV encephalopathy)
- Metabolic (Wilson’s disease)
- Parkinsonian syndromes in the context of other neurodegenerative conditions (atypical Parkinsonian syndromes)
- Multisystem atrophy
- Progressive supranuclear gaze palsy
- Corticobasal degeneration
- Lewy body dementia
Is Parkinson’s Genetic?
It is believed that about 10 to 15 percent of all Parkinson’s is genetic. The most common genetic mutations in genes linked with it include LRRK2, GBA, and SNCA.
LRRK2: Mutations in the LRRK2 gene have been shown to alter neurons. Genetic changes in this gene can be found in up to 2% of all people who develop the disease. At least 20 different gene mutations could occur in copies of this gene; some are more common, like G2019S. This is an autosomal dominant mutation, which requires inheriting the gene from one parent for the condition to occur.
GBA: This gene codes for a protein involved in removing cellular waste from cells. Between 5 to 10% of people with Parkinson’s have a genetic mutation in this gene. However, the chances that those who carry this mutation will develop Parkinson’s in the future is fairly low. Only a handful of mutations on the GBA gene have been linked to increased risk factors.
SNCA: This gene produces the protein alpha-synuclein. The brains of those with Parkinson’s contain clumps of this protein, called Lewy bodies. It is believed that genetic mutations in those with Parkinson’s can affect the SNCA gene and cause an excess amount of this protein in their brains, which then forms Lewy bodies and becomes toxic. Like LRRK2, this mutation is autosomal dominant.
Other genes, such as PARK7, PINK1, and PRKN are considered autosomal recessive mutations. In this case, two copies of the mutated gene must be present to cause the condition.
The Parkinson’s Foundation study, PD GENEration: Mapping the Future of Parkinson’s Disease, is the first national study to offer at-home genetic testing and counseling at no cost for those with a confirmed Parkinson’s diagnosis. Patients can also submit results from other genetic testing sites like Nebula Genomics, which offers 30X Whole Genome Sequencing.
Current Research on Genetic Parkinson’s
Living with PD can be challenging. However, there are organizations and support groups helping people enhance their quality of life. Many universities and scholars hold various studies that lead to a better understanding of genetic Parkinson’s. Initiatives like PD GENEration (mentioned above) offer genetic testing for patients for free.
We mentioned several genes associated with the development of PD. Furthermore, there was a 2017 research that studied that depression and catechol-O-methyltransferase (COMT) genetic variants are associated with pain in Parkinson’s disease. That same year, the University College of London and Northwestern University Feinberg School of Medicine presented a case report on Early-onset Parkinson’s Disease in a family of Moroccan origin caused by a p.A217D mutation in PINK1 to prove how history of family members with the condition increases risk.
Many alternative therapies have been developed. In 2020, a group of physicians from the Department of Neurosurgery, Massachusetts General Hospital reported the transplantation of induced pluripotent stem cells into the brain of a patient. This is the first human clinical trial.
The US government has dedicated funds to the study of Parkinson’s disease to try to help patients lead a satisfying life. The National Institute of Neurological Disorders and Strokes has developed the Parkinson’s Disease Biomarker Program to help improve research on new diagnostic and progression biomarkers for the condition.
In April 2021, US News reported the progress of Parkinson’s Disease. We are starting to see the light as technology is helping to the advancement of diagnostics and treatments.
Epidemiology
According to the Parkinson’s Foundation, More than 10 million people worldwide live with cases of Parkinson’s disease. According to their projections, nearly 1.2 million will be living with Parkinson’s in the United States by 2030, which is more than those diagnosed with muscular dystrophy, multiple sclerosis, and Lou Gehrig’s disease (or Amyotrophic Lateral Sclerosis) combined.
The disease affects close to 60,000 Americans each year.
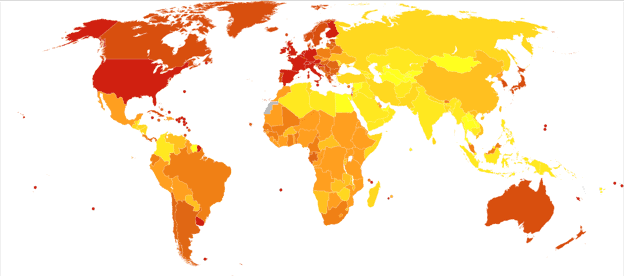
The incidence of Parkinson’s disease increases with age, but an estimated four percent of people with PD are diagnosed before age 50. Men are 1.5 times more likely to have Parkinson’s disease than women.
Symptoms
The condition begins gradually and gets worse over time. The symptoms become more severe as the condition progresses and are therefore more easily recognizable through daily activities. The most common form of Parkinson’s disease typically begins unilaterally, in one side of the body. Consequently, it is not uncommon for shoulder pain and unilateral muscle tension to occur, prompting the patient to first see an orthopedist.
According to the Parkinson’s Foundation, ten early signs of the condition include:
- Tremor
- Small handwriting
- Blood pressure changes
- Sense of smell loss
- Sleep problems (REM sleep behavior disorder)
- Trouble moving or walking
- Constipation
- Soft or low voice
- Hypomimia: Masked face
- Dizziness or fainting
- Balance issues
- Stooping or hunching over
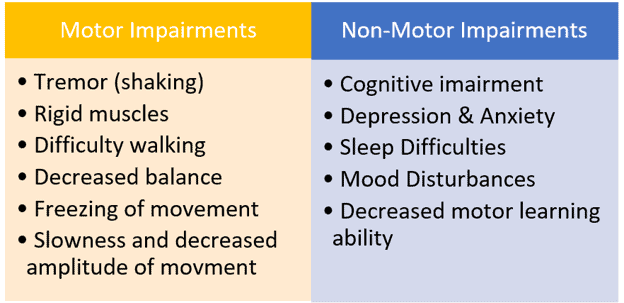
As the condition progresses, Parkinson’s disease is defined by the cardinal symptoms of bradykinesia (slow movement) or akinesia (loss or impairment of the power of voluntary action) and one of the other three leading symptoms (rigidity, tremor, postural instability).
Akinesia and/or bradykinesia
This general lack of movement is a prerequisite for the diagnosis of Parkinson’s disease. It is noticeable in all actions. Thus, the muscle play in the face is reduced, speech becomes quiet and unclear, swallowing is delayed, the dexterity of the hands decreases, especially with fast movements (handwriting becomes smaller), the trunk movements are difficult, and the gait becomes small-stepped and shuffling.
In Parkinson’s, this slowness happens in different ways:
- Reduction of automatic movements (such as blinking or swinging your arms when you walk)
- Difficulty initiating movements (like getting up out of a chair)
- General slowness in physical actions
- The appearance of abnormal stillness or a decrease in facial expression
Everyday activities such as eating, buttoning a shirt, or brushing your teeth become a challenge as a consequence.
Rigidity
This refers to muscle stiffness due to an increase in muscle tone. It is caused by an involuntary tensing of the entire striated musculature and often leads to muscle pain. Outwardly visible is a slight flexion of the elbow joint, trunk and neck, and later of the knee joints.
Tremor
The alternating tension of opposing muscles produces a relatively slow tremor that decreases with movement. The tremor is more prominent on one side of the body.
Postural instability
Decreased stability in holding the body upright occurs due to a disturbance in the positional reflexes. The small but rapid reflex compensatory movements are delayed, resulting in gait and stance instability. The turning movement becomes unsteady, and patients may start tripping.
The various Parkinson’s symptoms can be pronounced to different degrees in individual patients or be completely absent. Their occurrence and severity also change during the day.
Patients may also experience other illnesses such as low blood pressure and sleep problems.
Causes
A lot of people ask, “how do you get Parkinson’s disease?”
Scientists believe that a combination of genetic and environmental factors plays a role in the loss of dopamine in the brain and the following onset of Parkinson’s disease. The condition is very diverse among patients, with individuals differing in age onset, progression, and even treatment effectiveness. Family history is just one contributing factor.
Dopamine deficiency
Parkinson’s disease is a degenerative condition of the extrapyramidal motor system (EPS) or basal ganglia. It involves the death of nerve cells in the nervous system, which produces dopamine and transport it through their axons into the putamen. The first signs of the condition are only noticed when approx. 55% to 60% of these dopaminergic cells have died.
The dopamine deficiency ultimately leads to an imbalance in the function of the basal ganglia in two ways. The messenger substance glutamate is relatively abundant. The globus pallidus internus ultimately inhibits the motor activation of the cerebral cortex by the thalamus. This leads to the main symptoms of rigor, tremor, and hypokinesia, but also to a slowing of mental processes (bradyphrenia).
In addition to the dopamine deficiency, changes in other neurotransmitters have also been observed. For example, serotonin, acetylcholine, and norepinephrine deficiency was found in some regions of the brain stem.
Environmental Factors
Several environmental factors may lead to an increased risk of developing Parkinson’s, although scientific studies to confirm these associations have been largely inconsistent. These factors include:
- Head injury
- Exposure to metals
- Exposure to solvents and polychlorinated biphenyls (PCBs)
On the other hand, a strong link has been shown between PD and exposure to pesticides and herbicides. One such herbicide is paraquat, a commercial herbicide used in the U.S. banned in 32 countries, including the European Union and China.
Diagnosis
There is no specific test to diagnose Parkinson’s, and thus diagnosis is based solely on medical history, symptoms, and neurological examination. It may be difficult to diagnose Parkinson’s in its early stages when symptoms are minimal. While any medical provider may make an initial diagnosis, confirmation usually comes from a trained neurologist. Two of the four main motor symptoms must be present over some time:
- Shaking or tremor
- Bradykinesia: Slow movements
- Arms, legs, or trunk stiffness
- Postural instability: Struggle with balance and potential falls
Most tests such as blood tests and imaging tests may be performed to rule out other conditions that may be causing the symptoms.
Treatment
According to Mayo Clinic, treatment is based on individual symptoms. Treatments include a single or combination of medication, surgery, and lifestyle modifications, like physical therapy and exercise. It’s important to note that drugs are used to manage the symptoms, not cure the condition.
Medications
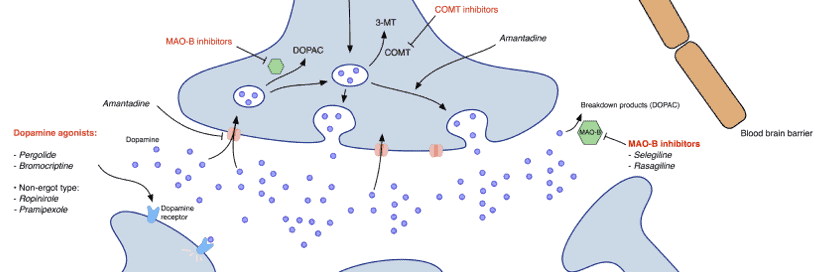
Most medications increase, substitute, or protect dopamine in the brain.
Carbidopa-levodopa: The most common medication is L-dopa (levodopa), a precursor of dopamine. This precursor, unlike dopamine itself, can cross the blood-brain barrier and be converted to dopamine in the brain. Levodopa is combined with carbidopa (Lodosyn) to protect it from early conversion to dopamine.
Newer forms of levodopa include Inbrija (inhaled form) and Duopa (infusion form).
Dopamine agonists: After several years of taking L-dopa, involuntary movements can occur. For this reason, treatment with a longer-acting dopamine agonist is usually recommended at the beginning of Parkinson’s disease, especially in younger patients. Instead of turning into dopamine, dopamine agonists are different drugs that mimic the action of dopamine and stimulate the dopamine receptors.
Although they aren’t as effective as levodopa, they last longer.
Dopamine agonists include ropinirole (Requip), pramipexole (Mirapex), and Rotigotine (Neupro, as a patch). Apomorphine (Apokyn) is a short-acting injectable dopamine agonist used for quick relief.
MAO B inhibitors: This medication helps prevent the breakdown of dopamine by inhibiting an enzyme that metabolizes brain dopamine.
COMT inhibitors: This medication mildly prolongs the effect of levodopa by blocking the enzyme that breaks down dopamine. When taken together with levodopa, they increase the availability of levodopa by 40 to 90 percent and prolong its plasma half-life. Medications in this category include entacapone and tolcapone.
Other medications: Other medications that are used more rarely are anticholinergics and amantadine. Anticholinergics used to be described to help control tremors. However, side effects include impaired memory and confusion. Patients may also experience hallucinations, constipation, dry mouth, and impaired urination, which have led to these medications not being prescribed as often anymore. Amantadine may provide short-term relief but some serious side effects like a purple mottling of the skin, ankle swelling, and hallucinations.
Surgery
Deep brain stimulation is a surgical procedure that may help relieve some of the symptoms of Parkinson’s disease. Surgeons implant electrodes into a specific part of your brain. The electrodes are connected to a generator implanted in your chest. Electrical pulses sent to your brain are used to reduce symptoms.
Like any surgery, deep brain stimulation entails risks that may include infections, strokes, or brain hemorrhage. And adjustments to the system may be necessary if a patient does not respond well to the stimulation.
Deep brain stimulation is most often offered to people with advanced Parkinson’s disease who have unstable medication responses. It is most helpful for controlling tremors. Deep brain stimulation can stabilize medication fluctuations, reduce or halt involuntary movements (dyskinesia), reduce tremors, reduce rigidity, and improve slowing of movement.
For additional information, clinical trial information, and treatment options, visit the Parkinson Foundation.
If you liked this article, you should check out our other posts in the Nebula Research Library!
July 1, 2022
