What is epigenetics?
Epigenetics is the study of factors that determine the activity of a gene in the human genome. It involves changes in gene function that are not based on changes in DNA sequences but passed on to daughter cells.
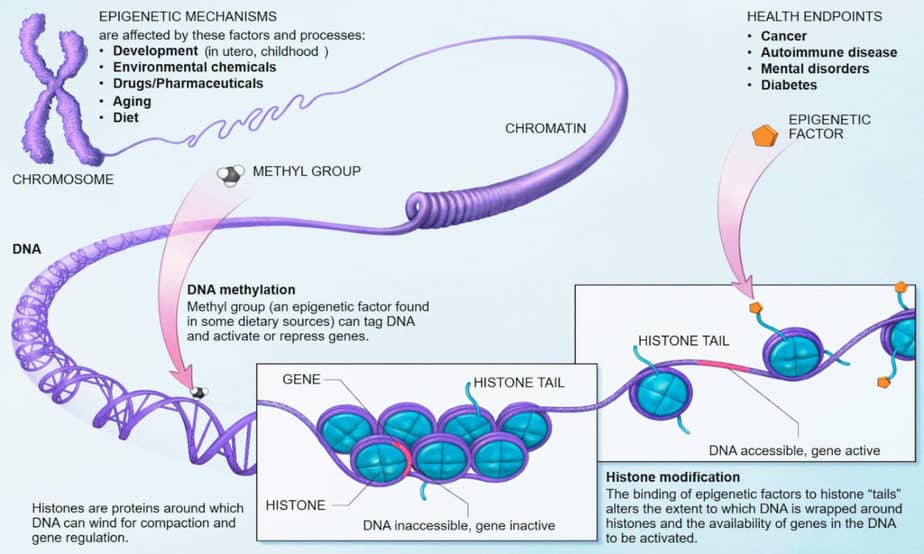
The basis for epigenetics are chemical changes in chromatin. It may affect the proteins that bind to DNA, or the DNA itself. These changes can influence the activity of sections or entire chromosomes. This is also referred to as epigenetic alteration or epigenetic imprinting.
As the DNA sequence is not changed, epigenetic modifications cannot be detected from the DNA sequence. Types of epigenetic processes include inactivating X chromosomes, imprinting gene, or storing the transcriptional memory of cells.
Edited by Christina Swords, Ph.D.
Epigenetics basics
After fertilization, the egg cell divides. Up to the 8-cell stage, all daughter cells are equal. They are called totipotent because each of them is still capable of producing a complete organism on its own.
After this stage, there are cells with a different internal program. These cells have limited development potential as it becomes more and more specialized.
When the body is fully formed, most body cells are firmly programmed for their function based on epigenetic mechanisms. The function was fixed due to biochemical modifications of the bases in DNA or the histones packaging the DNA, or both. The sequence of the genetic material remains unchanged apart from a few random mutations.
Such epigenetic modifications result in certain regions of the genome being “silenced”, i.e. cannot be easily transcribed into RNA for protein synthesis. These modifications look quite different in somatic cells than in stem cells or in germ cells. The most important modifications are the methylation of cytosine bases and the side-chain methylation and acetylation of histones.
Besides methylation, telomeres have an important epigenetic influence. Telomeres protect the ends of chromosomes from degradation during cell division. The enzyme telomerase ensures that the chromosomes remain intact. Mental stress can reduce the activity of this enzyme, ultimately led to an accelerated shortening of telomeres in the aging process.
Comparison to genetics
The term epigenetics can be understood when one considers the process of inheritance:
- Before a cell divides, the genetic material is doubled. Half of the duplicated genome is then transferred to one of the two daughter cells. Half of the maternal genetic material is brought by the egg cell whereas the paternal half by the sperm cell.
- Molecular genetics describes the genetic material as a double helix of two deoxyribonucleic acid strands. The strands have phosphate-deoxyribose sugar polymer as backbones. The genetic information comes from the sequence of the four bases that are attached to the deoxyribose sugar backbone. Those are adenine (A), cytosine (C), guanine (G) and thymine (T).
- The bases of one strand almost always pair with a matching base of the second strand. Adenine pairs with thymine, and cytosine pairs with guanine.
- The genetic information is anchored in the order of the building blocks A, C, G, T (the base sequence).
Some phenomena of heredity cannot be explained with the DNA model just described:
- During cell differentiation, daughter cells with a different function are formed in the course of cell division. After cell differentiation, different cell types still retain similar DNA sequences. The determination of the functional identity of a cell is a topic of epigenetics.
- There are traits that are only “inherited” from the father or the mother and are not related to the DNA. Disturbances of this state lead to serious diseases.
- When differentiated cells are transformed back into stem cells, epigenetic patterns must be removed. A cell can once again acquire and inherit all or many functions when epigenetic fixations are removed.
Histones and their role in epigenetic fixation
DNA is not present naked in the cell nucleus, but is bound to histones. Eight different histone proteins form the nucleus of a nucleosome on which 146 base pairs of a DNA strand are wound. The ends of the histone strands protrude from the nucleosome and are the target of histone-modifying enzymes.
Histone modifications are mainly methylation and acetylation on lysine, histidine or arginine, as well as phosphorylations on serines. It also plays a role whether the lysine side chain is occupied by one, two or three methyl groups. A kind of “histone code” may be related to the activity of the genes bound by the histones.

Influence of methylation and acetylation on the conformation of chromatin
Changes in the histone side chains change the volume of a gene segment. There are smaller volumes of gene segments in closed conformation, along with chromosome condensation and gene inactivation. Larger volumes of gene segments can be observed in open conformation along with gene activity. Transition between the two states is caused by attachment and cleavage of methyl groups to cytosine bases.
In general, the attachment of acetyl groups to the histones leads to the opening of the nucleosome conformation. This opening makes the gene available for transcription by the RNA polymerase. Attachment of methyl groups to lysine side chains leads to the attachment of methyl-binding protein MeCB that suppresses gene expression. These repressor proteins close the histone’s conformation, preventing transcription.
Epigenetic changes in the life course
Epigenetics is not limited to heredity cases. Increasing attention is being paid to the connection between ongoing changes in the life course and the development of diseases. For example, identical twins show a high degree of epigenetic similarity at the age of three but not 50. The degree of methylation was up to 2.5x higher in one twin.
Thus, despite their genetic identity, older twins are epigenetically more diverse the more different the life course of the twins is. Not only the environment they experience causes this, but also the inaccuracy of methyl group patterns transmission during each cell division. Gradual changes thus add up more and more in the course of a lifetime.
The change of diet in worker bees causes a highly epigenetic reprogramming of the larval genome. More than 500 genes have been identified that are affected by the environmentally induced methylation changes.
Activation or non-activation of genes is not the only consequence of dietary change. There are also alternative splicing and altered gene products.
Epigenetic changes as an explanation of diseases
The explanation of stress factors is a major focus of epigenetic research. Individuals with early traumatic life experiences, triggered by lack of maternity care, have been used for this purpose. Stress triggers a cascade of hormone which begins in the hypothalamus, a part of the diencephalon.
It has been shown that a glucocorticoid gene shows strikingly different methylations in the individuals concerned. Accordingly, the gene is inhibited in the presence of a history of stress. The gene product in the adrenal cortex, the terminal station of the hormone chain, is subsequently different. More than 900 genes are up- or downregulated in the brain as a result of maternal behaviour.
The results have also been confirmed in humans. The receptor gene in the hippocampus in humans is largely identical to that of other mammals. Epigenetic changes are therefore similar to those in rats.
A study with suicide candidates divided affected persons into two groups, those with childhood abuse experiences and those without. Only in the candidates with a history of abuse was the receptor gene blocked with methylation.
A trauma experienced by the mother during pregnancy can even have lasting consequences for the expectant child that last for decades. There is a significant increase in risk of schizophrenia and heart disease in children from mothers who suffered from famine. These children have also been observed to carry changes in the methylation pattern of the Igf2 gene.
In mice, regular coca consumption changes the epigenetic pattern of several hundred genes in the reward centre of the brain. This increases sensitivity to drug effects and increases the risk of addiction.
The magnitude of epigenetic changes over the course of a lifetime is many times greater than that of genetic mutations. It may provide new answers to a variety of diseases such as schizophrenia, Alzheimer’s disease (plus disorders related to Alzheimer’s like dementia), cancer, adult-onset diabetes, nervous disorders, and many more.
Inheritance of epigenetic imprints
The findings on epigenetic alterations, especially in popular science, are constantly drawing parallels with Lamarckism. Lamarckism, or the passing of acquired characters, is seen as contradictory to classical genetics.
However, there is very little evidence that learned and acquired abilities can be passed on via the germ cells. Passing on acquired abilities to the following generation is not yet proof of genetic manifestation. Also, the term “generation” is often misinterpreted as the beginning of an individual cycle.
An inheritance of epigenetic imprints was proposed in 2003 by Randy Jirtle and Robert Waterland using mouse experiments. Female agouti mice were administered a certain composition of nutrients before mating and during pregnancy. It was found that a large proportion of the offspring did not exhibit the typical phenotype.
In a human study, various factors that provided information on food availability and mortality in the small Swedish town of Överkalix. It was found that most people whose grandparents had sharp dietary changes developed cardiovascular diseases as they grew older.
However, the disease followed a certain pattern, which suggests epigenetic changes on the sex chromosomes. For example, in families where the grandfather had eaten well or eaten too much, only the male grandchildren were affected.
According to a hypothesis by William R. Rice and colleagues, epigenetics may be the cause of human homosexuality. The sexual preference of the mother would be transmitted to the son and the preference of the father to the daughter.
This would happen if the epigenetic markers on genes responsible for sexual orientation were retained in the germ cells. If the epigenetic marks in the egg cell are not removed, the embryo can have the sexual orientation of the mother. According to this hypothesis, homosexuality in humans is innate.
The hypothesis explains why the occurrence of homosexuality in humans remains statistically stable over time. However, there is no empirical evidence for a connection between homosexuality and epigenetics.
